Clinical Information
The following information is intended for healthcare practitioners, and consumers who want more details on the performance and specification of the MonitorYou analytical services.
HbA1c
The MonitorYou fingerprick blood analysis method for HbA1c was developed by researchers from the Australian National University and aims to improve the access and convenience of blood monitoring for risk of chronic conditions and to maintain general wellness. Their study published in BMC Clinical Pathology in 2015 found comparable results between the MonitorYou Diabetes HbA1c dried blood spot analysis and traditional whole blood testing.
Improvements to the published HbA1c fingerprick method were implemented and demonstrated stability of results up to 11 days post sample collection, eliminating the need for application of correction formulae.
Dried blood spot technology
Dried blood spot (DBS) results obtained for HbA1c are comparable to whole blood samples collected by venepuncture and analysed by the same method (Indiko Immunoturbidimetric), and demonstrate clinically acceptable precision to be used as a tool for monitoring diabetes risk.
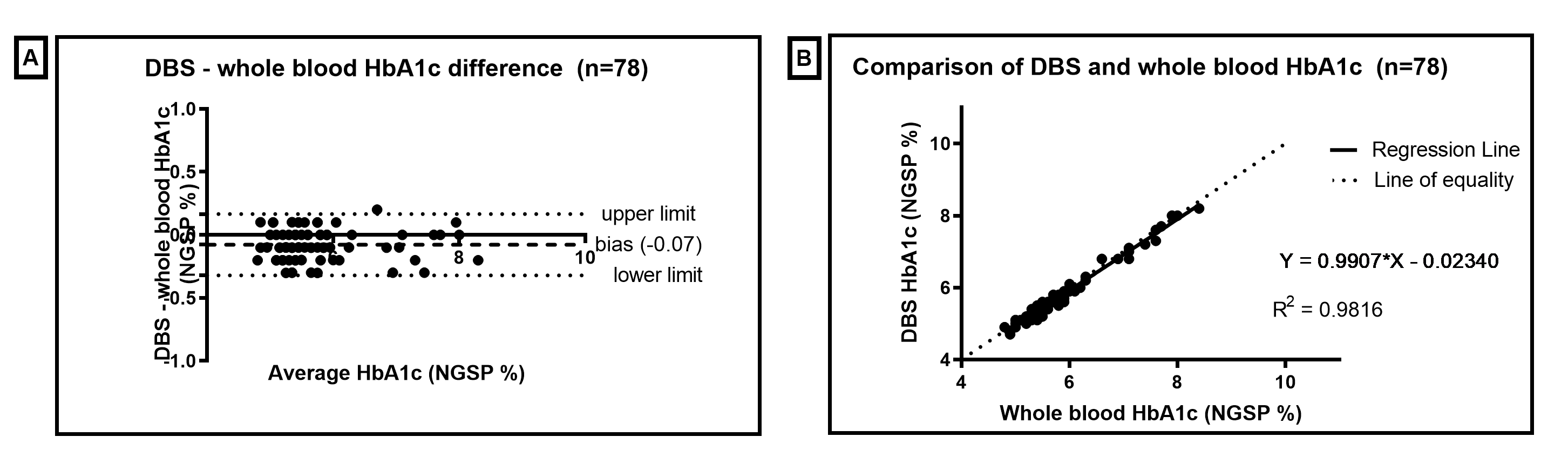
Figure 1. HbA1c Bland Altman and Linear Regression of DBS compared to whole blood
| Assay Type: |
Immunoturbidimetric (Thermo Fisher Scientific) |
||||||
| Instrument: |
Indiko (Thermo Fisher Scientific) |
||||||
| Sample: |
Fingerprick dried blood spot (DBS) |
||||||
| Measurand: |
HbA1c concentration in whole blood |
||||||
| Measurement Range: |
4-15% NGSP** 20-140 mmol/mol IFCC# (at a normal haemoglobin (Hb) concentration of 150 g/L) |
||||||
| Reference Interval: |
|
||||||
| Repeatability* |
3.4% CV at 6.1% HbA1c (NGSP), 1.5% CV at 10.6% HbA1c (NGSP) |
||||||
| Stability: |
up to 11 days |
||||||
| Interferences: |
Samples containing high amounts of glycated fetal haemoglobin (HbF) >10% may result in lower HbA1c values than expected. The TAS HbA1c does not detect rare cases of Hb Raleigh. |
||||||
| Thermal stability: |
Research has shown that after holding dried blood spot samples at 46°C for 2 days there is a slight increase in the HbA1c level from people with an HbA1c of 7.5% and higher. Therefore, there may be a small variation in results if samples are subjected to extreme temperature conditions. |
**National Glycohemoglobin Standardization Program
#International Federation of Clinical Chemistry
*Further information on measurement uncertainty is available upon request.
What can affect the results?
When evaluating HbA1c results there are several factors that need to be considered by health professionals.
Medications: some medications may cause a rise in HbA1c results because they predispose the individual to higher blood glucose levels. These include corticosteroids, antipsychotic drugs and antiretrovirals. Other medications can change HbA1c results because they affect red blood cell turnover. In these cases, HbA1c may not accurately reflect glycaemic status. An example is the drug Dapsone, which can cause falsely low HbA1c results by increasing red blood cell turnover.
Some medications, like Dapsone and Aspirin, may affect the results of dried blood spot testing more generally, because they can change your red blood cell count.
Medical considerations: certain factors should be considered as they are known to affect HbA1c levels. These include conditions that alter red blood cell survival or cause red blood cell destruction, conditions that change the glycosylation rate of HbA1c and conditions in which haemoglobin itself is altered. In the presence of these conditions, HbA1c may not accurately reflect glycaemic status. These include:
- Deficiency in iron or vitamin B12
- Kidney failure
- Anaemia
- Chronic liver disease
- Conditions affecting the spleen or pancreas
- Genetic haemoglobin defects
- Rheumatoid arthritis
- Alcoholism
This list is not exhaustive.
hsCRP
The MonitorYou fingerprick blood hsCRP analysis method was developed and validated following National Pathology Accreditation Council (NPAAC) criteria and aims to improve the access and convenience of blood monitoring for disease prevention. Our validation found comparable results between the hsCRP dried blood spot analysis and traditional serum testing.
Dried Blood Spot Technology
Dried blood spot (DBS) results obtained for hsCRP are comparable to serum samples collected by venepuncture and analysed by the same method (ELISA), and demonstrate clinically acceptable precision to be used as a tool for monitoring hsCRP levels.
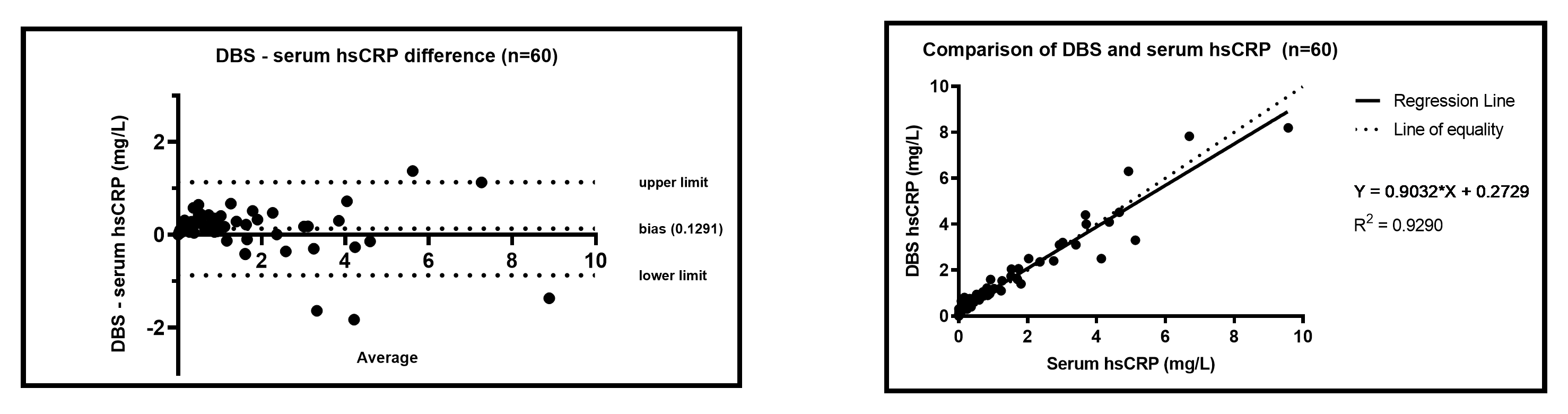
Figure 1. hsCRP Bland Altman and Linear Regression of DBS compared to serum
| Assay Type: |
ELISA (DRG International) |
||||||||
| Instrument: |
Enspire spectrophotometer (Perkin Elmer) |
||||||||
| Sample: |
Fingerprick dried blood spot (DBS) |
||||||||
| Measurand: |
hsCRP concentration in whole blood |
||||||||
| Measurement Range: |
0.4 mg/L to 10 mg/L |
||||||||
| Reference Interval: |
|
||||||||
| Repeatability* |
11% CV at 0.99 mg/L, 6% CV at 5.9 mg/L |
||||||||
| Stability: |
20 days |
||||||||
| Interferences: |
Haematocrit values outside normal range of 39 – 53% may affect hsCRP values as a result of non-uniform spreading of blood on the collection card. |
||||||||
| Thermal stability: |
Testing has shown that hsCRP is stable after holding dried blood spot samples at 46°C for 2 days. |
*Further information on measurement uncertainty is available upon request.
What can affect the results?
When evaluating hsCRP results there are several factors that need to be considered by health professionals.
Medications: the following medications may affect results.
- Non-steroidal anti-inflammatory drugs (aspirin, dapsone, ibuprofen, rofecoxib, celecoxib, and naproxen)
- Statins (atorvastatin, rosuvastatin)
- Platelet aggregation inhibitors (clopidogrel, abciximab)
- ACE inhibitors (ramipril, captopril, fosinopril)
- Colchicine, which is used to treat gout
- The oral contraceptive pill
- Hormone replacement therapy
Some medications, like Dapsone and Aspirin, may affect the results of dried blood spot testing more generally, because they can change your red blood cell count.
This list is not exhaustive.
Medical considerations: certain conditions should be considered as they are known to affect hsCRP levels. These include causes of acute inflammation such as:
- Injury
- Bacterial and viral infections
- Autoimmune diseases such as rheumatoid arthritis and lupus
- Inflammatory bowel disease
- Obesity
- Advanced cancer
Triglycerides
The MonitorYou fingerprick blood triglycerides analysis method was developed and validated following National Pathology Accreditation Council (NPAAC) criteria and aims to improve the access and convenience of blood monitoring for disease prevention. Our validation found comparable results between the Triglycerides dried blood spot analysis and traditional serum testing.
Dried Blood Spot Technology
Dried blood spot (DBS) results obtained for Triglycerides are comparable to serum samples collected by venepuncture and analysed by the same method (Enzymatic), and demonstrate clinically acceptable precision to be used as a tool for monitoring triglyceride levels.
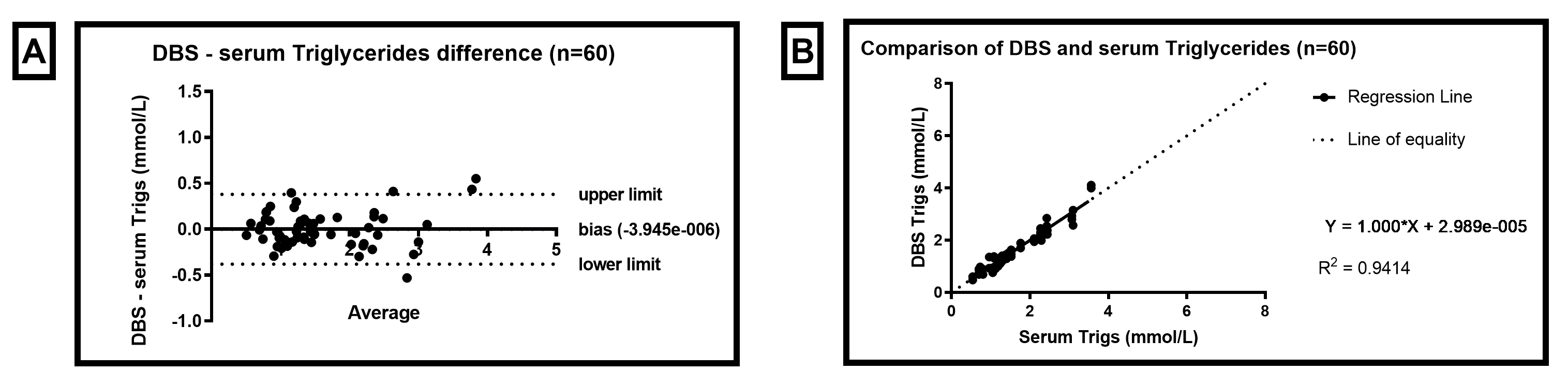
Figure 1. Triglyceride Bland Altman and Linear Regression of DBS compared to serum
| Assay Type: |
Enzymatic (Randox) |
||||
| Instrument: |
Enspire spectrophotometer (Perkin Elmer) |
||||
| Sample: |
Fingerprick dried blood spot (DBS) |
||||
| Measurand: |
triglycerides concentration in whole blood |
||||
| Measurement Range: |
0.2 mmol/L to 11.4 mmol/L |
||||
| Reference Interval: |
|
||||
| Repeatability* |
4.1% CV at 1.1 mmol/L, 2.9% CV at 4.1 mmol/L |
||||
| Stability: |
20 days |
||||
| Interferences: |
Topical application of creams containing glycerol at the site of sampling. Haematocrit values outside normal range of 39 – 53% may affect Trig values as a result of non-uniform spreading of blood on the collection card |
||||
| Thermal stability: |
Testing has shown that triglycerides are stable after holding dried blood spot samples at 46°C for 2 days |
*Further information on measurement uncertainty is available upon request.
What can affect the results?
When evaluating triglycerides results there are several factors that need to be considered by health professionals.
Medications: the following medications may affect results by increasing triglyceride levels.
- Thiazide diuretics
- Antipsychotics
- Beta-blockers
- Corticosteroids
- Anabolic steroids
- Anticonvulsants
- Protease inhibitors
- Immune-suppressive agents such as cyclosporine
- The oral contraceptive pill
- Isotretinoin
- Growth hormone
Some medications, like Dapsone and Aspirin, may affect the results of dried blood spot testing more generally, because they can change your red blood cell count.
This list is not exhaustive.
Medical considerations: certain medical conditions should be considered as they are known to affect triglyceride levels. These include:
- Poorly controlled diabetes
- Hypothyroidism and hyperthyroidism
- Cirrhosis
- Kidney disease
- Malnutrition
Omega fatty acids
The MonitorYou fingerprick blood Omega fatty acid analysis method was developed in collaboration with researchers from the South Australian Health and Medical Research Institute (SAHMRI) and validated following National Pathology Accreditation Council (NPAAC) criteria and aims to improve the access and convenience of blood monitoring for disease prevention. Our validation found comparable results between the Omega fatty acid dried blood spot analysis and traditional whole blood testing.
Dried Blood Spot Technology
Dried blood spot (DBS) results obtained for Omega Fatty acids are comparable to whole blood samples collected by venepuncture and analysed by the same method (GC-MS/MS) and demonstrate clinically acceptable precision to be used as a tool for monitoring Omega fatty acid levels.
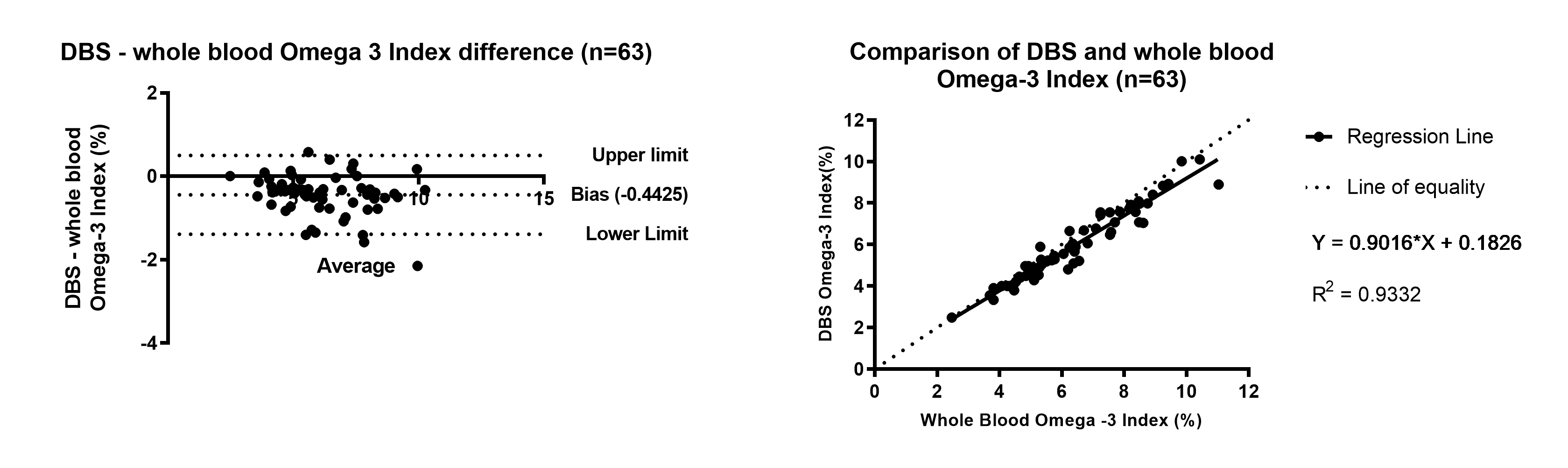
Figure 1. Omega-3 index Bland Altman and Linear Regression of DBS compared to whole blood
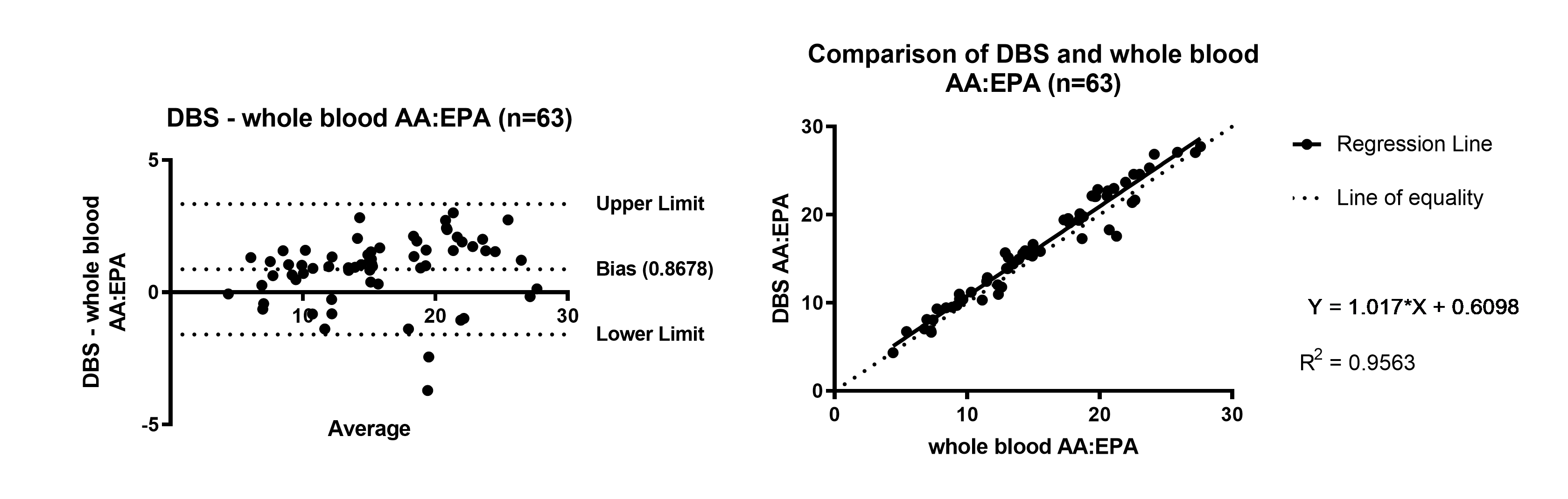
Figure 2. AA:EPA ratio Bland Altman and Linear Regression of DBS compared to whole blood
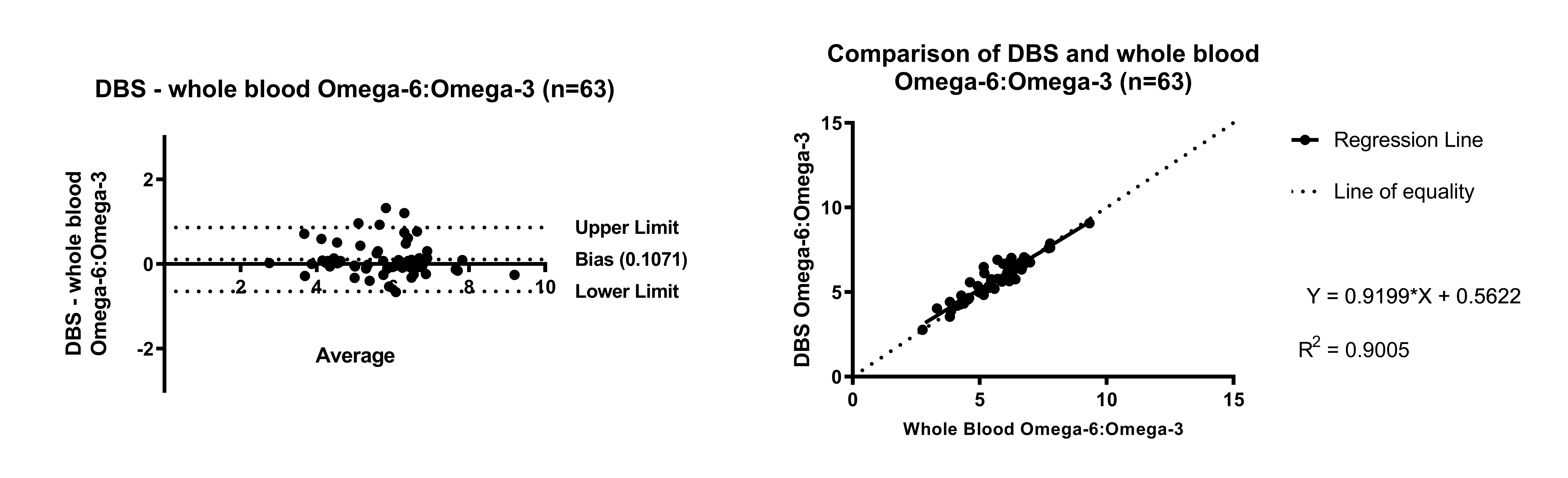
Figure 3. Omega-6:Omega-3 ratio Bland Altman and Linear Regression of DBS compared to whole blood
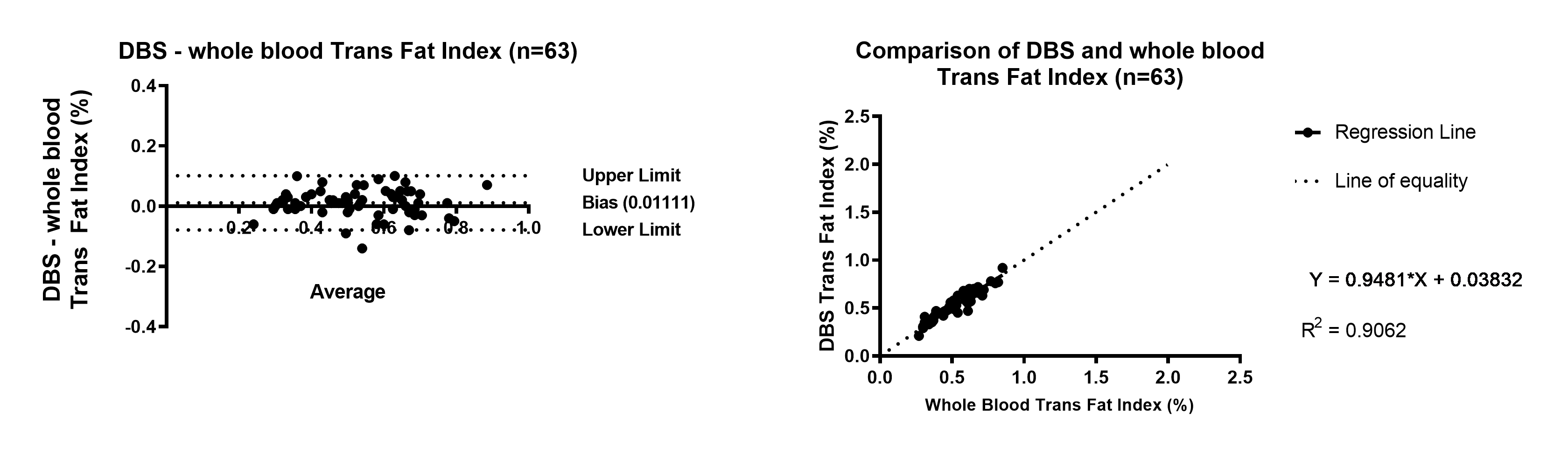
Figure 4. Trans Fat Index Bland Altman and Linear Regression of DBS compared to whole blood
| Assay Type: |
GC-MS/MS |
| Instrument: |
Thermo Fisher GC-MS/MS |
| Sample: |
Fingerprick dried blood spot (DBS) |
| Measurement Range: |
Dependent on the absolute value of individual FAs |
| Measurand: |
Omega-3 index (whole blood) AA:EPA ratio (whole blood) Omega-6 : Omega-3 ratio (whole blood) Trans fats index (whole blood) |
| Reference Interval: |
< 4.0% - Low Omega-3 4.0 – 8.0% - Acceptable, room for improvement > 8.0% - Optimal ≤ 1.5:1 – Optimal Between 1.5:1 – 10:1 – Acceptable, room for improvement ≥ 10:1 - High 3:1 – 5:1 - Optimal < 3:1 - Moderately low 5.1:1 – 9:1 - Moderately high > 9:1 - High ≤ 1.0% - Optimal > 1.0% - Trans Fat intake is high |
| Repeatability* |
2.2% CV at 8.2% 1.1% CV at 6.5:1 2.0% CV at 4.2:1 5.8% CV at 0.74% |
| Stability: |
15 days |
| Interferences: |
No interferences reported |
| Thermal stability: |
Testing has shown that Omega Fatty acids are stable after holding dried blood spot samples at 46°C for 3 days at room temperature. |
*Further information on measurement uncertainty is available upon request.
What can affect the results?
When evaluating omega fatty acid results, several factors need to be considered by health professionals. Omega fatty acid levels are largely influenced by the amount of fatty acids in the diet so measurements are generally more reflective of long-term fatty acid intake.
Medications: there are very few medications known to influence omega fatty acid results. Non-steroidal anti-inflammatory drugs such as aspirin, ibuprofen and indomethacin may influence the results.
Some medications, like Dapsone and Aspirin, may affect the results of dried blood spot testing more generally, because they can change your red blood cell count.
Medical considerations: Certain medical conditions should be considered as they are known to affect omega fatty acid levels. These include chronic malnutrition, and diseases of malabsorption such as pancreatic insufficiency, cystic fibrosis and inflammatory bowel disease. Conditions of high levels of inflammation (such as autoimmune diseases) may also lower the results.



